What Is The Molecule Geometry Of Clf3
The molecular geometry of chlorine trifluoride is T-shaped. These are arranged in a trigonal bipyramidal shape with a 175 Faxial-Cl-Faxial bond angle.
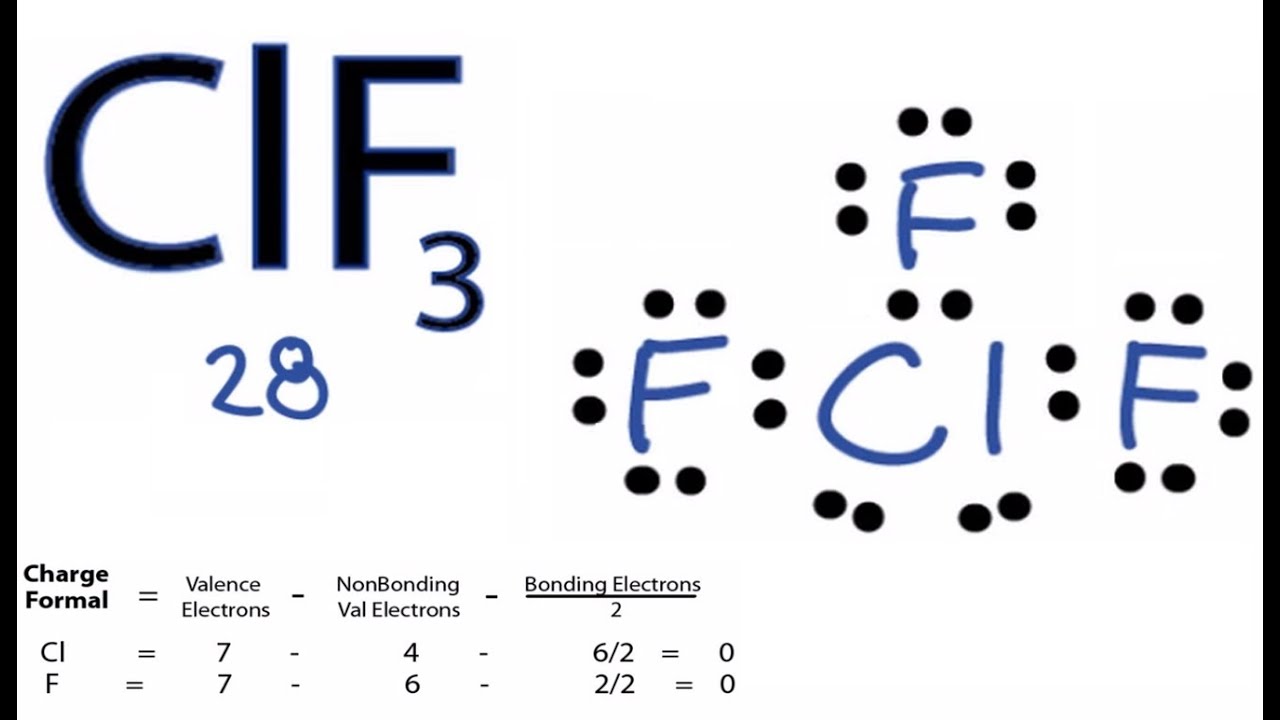
Clf3 Chlorine Trifluoride Molecular Geometry Bond Angles Electron Geometry Youtube
It acquires such shape because of the.
What is the molecule geometry of clf3. Contact with organic materials may result in spontaneous ignition. ClF3 Chlorine Trifluoride These are arranged in a trigonal bipyramidal shape with a 175 F axial-Cl-F axial bond angle. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom 3 bonds and 2 lone pairs.
As a result the bond angle of Cl-P-Cl gets deviated and is less than 109 degrees. These cookies do not store any personal information. Determine the molecular geometry of ClF3.
Want to see the step-by-step answer. Label all bond angles in the structure. FREE Expert Solution Show answer.
There are two equatorial lone pairs making the final structure T shaped. Learn this topic by watching Electron Geometry Concept Videos. Back to Molecular Geometries Polarity Tutorial.
92 214 ratings Problem Details. Two F atoms occupy the axial positions and one F occupy the equitorial positions of trigonal bipyramidal arrangement. The molecular geometry of chlorine trifluoride is T-shaped.
What is the molecular geometry of clf3. The molecular geometry of ClF 3 is T-shaped with asymmetric charge distribution around the central atom. What is the molecular geometry of ClF3.
Thank you so much to whom ever will answer my confusing thoughts right now. Molecular Geometry Polarity Tutorial. It reacts with water to form chlorine and hydrofluoric acid with release of heat.
This means there are five electron pairs arranged in a trigonal bipyramidal shape with a 175 F C lF bond angle. The result is a T-shaped molecule. ClF3 shows Trigonal bipyramidal geometry.
Following the octet rule. But it is not so I dont know what it should be named in molecular geometry. Chlorine trifluoride has 10 electrons around the central chlorine atom.
8 rows The molecular geometry of ClF3 is T-shaped and electron geometry is Trigonal bipyramidal. An interhalogen compound having both Cl and F it has a density of around 379 gl and a molar mass of 9245 gmol. The two lone pairs take equatorial positions because they demand more space than the bonds.
See full answer below. All Chemistry Practice Problems Electron Geometry. As per the molecular geometry of the molecule the bond angle of PCl3 should be 109 degrees.
Answer verified by Toppr. But as there is one lone pair of electrons on the central phosphorus atom the bond angle will reduce from 109 degrees because of the repulsive forces of the lone pair. Label all bond angles in the structure.
The two lone pairs take equatorial positions because they demand more space than the bonds. The hybridisation of ClF3 is sp3d and possess 3 bond pairs and 2 lone pairs. What is the molecular geometry of ClF3.
The result is a T-shaped molecule. It possess shape as T - shape. Determine the molecular geometry of ClF 3.
ClF3 Lewis Structure Molecular Geometry Hybridization and Polarity Chlorine trifluoride or ClF3 is an extremely reactive chemical compound with several varied applications and unique physical and chemical compounds. Chlorine Trifluoride on Wikipedia. If two bonds of trigonal biyramidal basic geometry are changed into two.
Start your trial now. 5 rows ClF3 molecular geometry is said to be a T-shaped. It took 753 ml of 0150 M NaOH to neutralize the acid.
First week only 499. The shape of ClF3 molecule is T shaped The hybridisation is Sp3d. Hi I thought that ClF3 is trigonal bipyramid or triangular bipyramidal.
The central atom chlorine is attached to three fluorine atoms. Therefore ClF3 is polar. If central atoms contains 5 bond repulsion units and if it doesnt contain lone pair on central atom the shape of the molecule is trigonal bipyramidal.
Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor.

Clf3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Clf3 Lewis Structure Molecular Geometry Shape Is It Polar Or Non Polar

Hybridization Of Clf3 Hybridization Of Cl In Chlorine Trifluoride
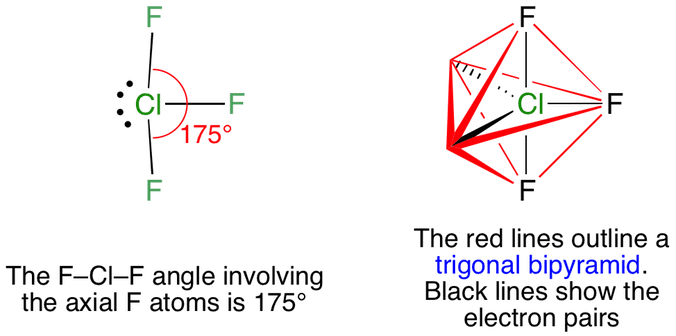
Vsepr Clf3 Chlorine Trifluoride

What Is The Molecular Geometry Of Clf3 Quora

What Is Molecular Geometry Of Ab5 And Ab6 Type Compounds

Clf3 Chlorine Trifluoride Molecular Geometry Bond Angles Electron Geometry Youtube

Hybridization Of Clf3 Hybridization Of Cl In Chlorine Trifluoride
0 Response to "What Is The Molecule Geometry Of Clf3"
Post a Comment